Avoid Secondary Surgery Biodegradable Intestinal Bypass Stents
PRODUCT FEATURES

PRODUCT DESCRIPTION
Product Use: |
Applicable for bowel bypass surgery. |
Product Highlights: |
It is the first medical device in the world to protect the anastomotic stoma of low rectal cancer from stoma and reposition surgery. It can disintegrate and excrete from the body within 30 days. |
Device Classification: |
[CN] Class II |
Material: |
PGA, Barium Sulfate |
Specification Description: |
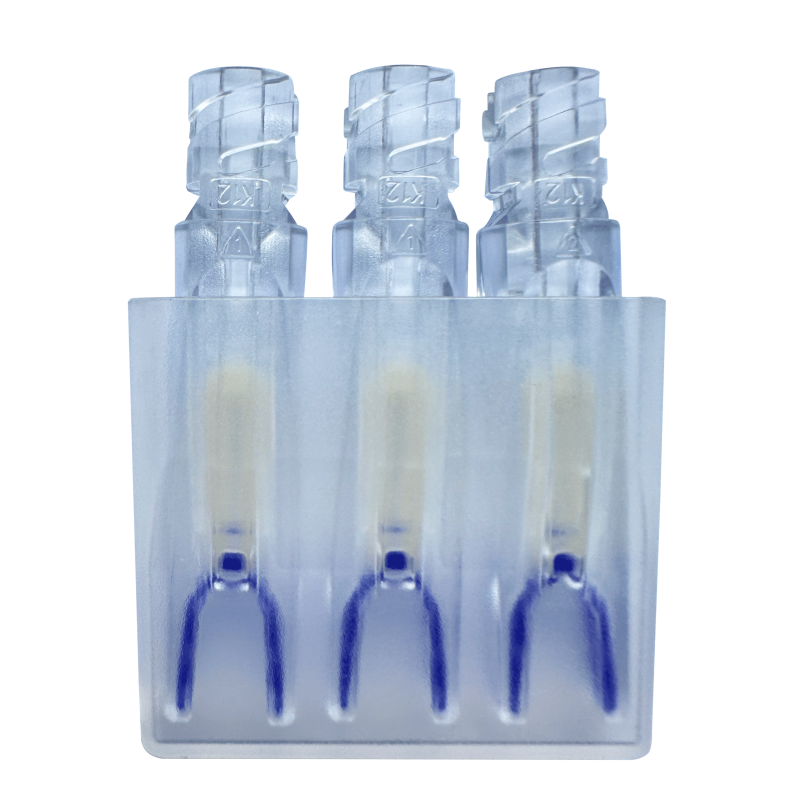
Different specifications represent the maximum diameters of anastomotic stents of 22 mm, 24 mm……38 mm |
Storage Conditions: |
The product shall be stored in an environment that is cool, dry, well-ventilated, clean with a relative humidity of not more than 80% and free of corrosive gases to avoid prolonged exposure to elevated temperatures. Do not expose the device to an environment over 40℃. |
Product Specification
Instructions: |
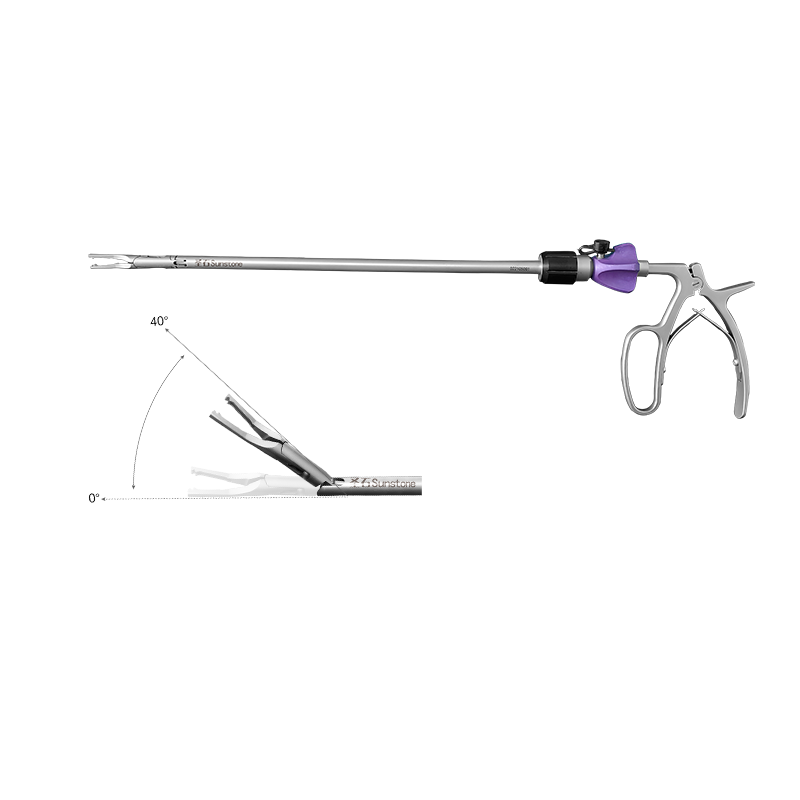
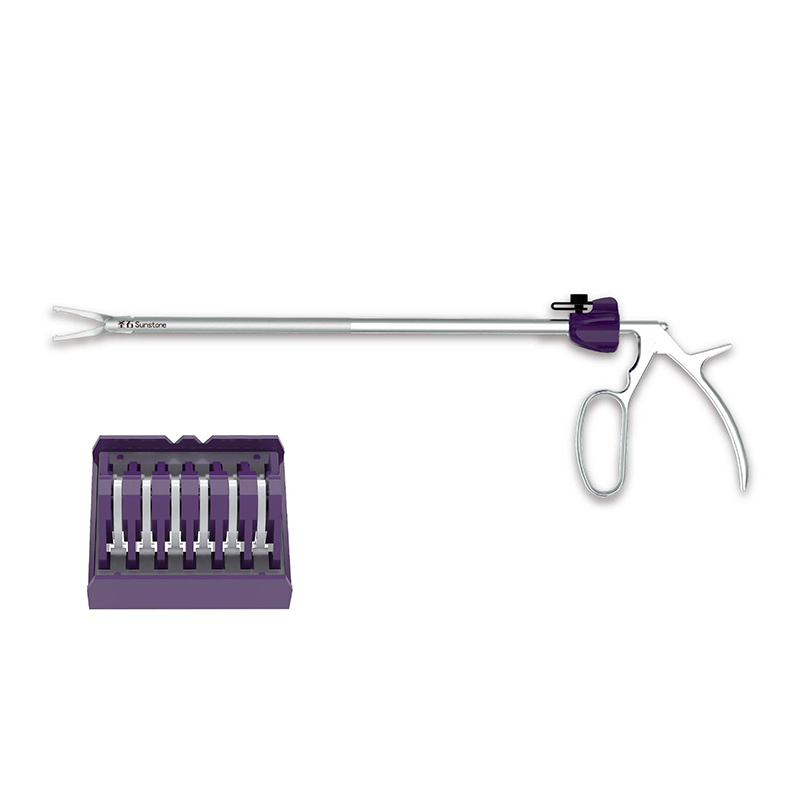
Create an ileal incision 10 cm-15 cm from the ileocecal junction, place a product with appropriate size, and finally perform suture. Then place a drainage tube in the incision 10 cm proximal to the anastomosis. See the IFU for details. |
Product advantages: |
Effective prevention of anastomotic leakage; It can avoid artificial anus indwelling and secondary operation; Short treatment duration (2-4 weeks) Within 4 weeks after surgery, the product can gradually disintegrate in the intestinal tract and excrete from the body, without residual foreign matters; The product contains barium sulfate, which can be developed under X-ray, so its disintegration process is allowed to be dynamically tracked; The physical and psychological trauma of the patients is significantly reduced. |
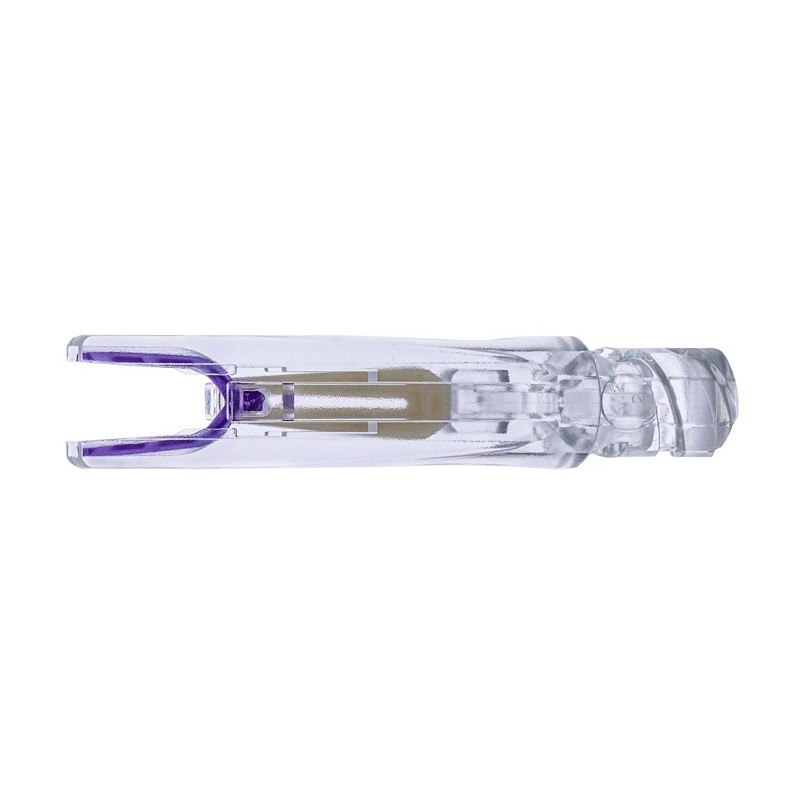
Product structure and composition: |
One-piece anastomosis stent |
Precautions: |
The product is for single use; it is not designed to be used in case of damaged package. The product must not be used by unskilled or untrained personnel. It is contraindicated in patients requiring two or more intestinal anastomoses. |
Shelf life: |
2years |